

2024
- Completed GMP certification (Product category: Human tissue or functional substitutes).
- Signed an exclusive distribution agreement for China with Nanjing Ruimeiaishe Medical Technology Co., Ltd. for Sina System.
- Entered into an exclusive supply and joint product development agreement for Silk Fibroin with Favorsun medical Tech. (suzhou) Co., Ltd in China.


2023
- Designated as a 'Global Small Giant 1000+' by the Ministry of SMEs and Startups.
- Obtained and filed domestic and international patents for the animal medical device, Planicare, utilizing plasma technology.
- Planicare was honored with the Good Design Award.
- Established the GCS Corporate Research Institute (BIO Research Center).


2022
- Relocated and expanded the GCS factory and facilities.
- Awarded the Minister of SMEs and Startups Prize (Export Innovation Enterprise Award).
- Achieved the $10 Million Export Tower Award.


2021
- GANA FILL GOST-R Certification acquired
- INOBELLE+D (Lidocaine) CE Certification acquired
- Brazilian partner firm Innovapharma launched PLLA filler brand ‘Rennova Elleva’
- Launched online shopping mall ‘Clinic At Home’
- Leaf
- ·Publication of its dissertation in the academic journal SCI 1Q
- ·Awarded 1st place in the beauty device category of the 2021 Korean consumer satisfaction awards
- ·Launched Leaf Homecare Device Package for LOTTE home shopping
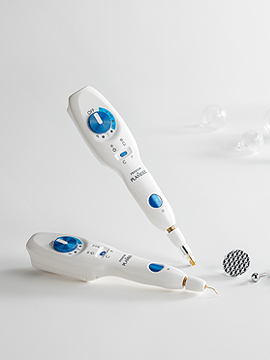

2020
- Established G Bio Lab
- Supply contract with Sinclair Pharma for ‘PLLA Filler’
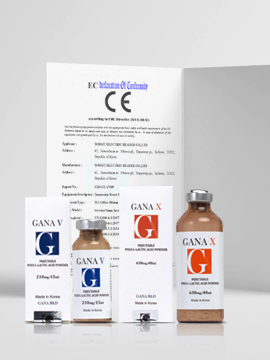
- GANA FILL ‘ANVISA’ certification acquired
- Upgraded and launched the PREMIUM PLAMERE
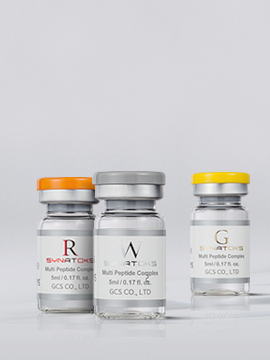
- Launched SYNATOKS
- Launched SYNA M, skinp
2019
- GANA Fill medical-use CE acquired
- Developed 3-type SYNATOKS
- Leaf registered patent application (skincare device and
the operating method of the device)- Art Lift Russian medical device certification GOST-R acquired


2018
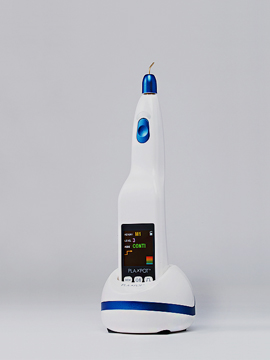
- PLAXPOT medical-use CE acquired
- Launched Leaf, plasma homecare beauty device
using medical plasma technology- GANA FILL registered patent application (Manufacturing method for PLLA filler and hyaluronic acid filler composite body)
- PLAXPOT(medical plasma device) medical CE acquired
- PLAMERE (skin-specific electric device and
the operating method of the device) patent acquired- Launched multi-peptide hyaluronic-acid cosmetic MTP36
- Opened Seoul office

2017
- Launched cross-link hyaluronic-acid filler INOBELLE+
- Launched medical-use plasma device PLAXPOT / formal registration in
Korea as a medical device- Registered PLAXPOT (skin-specific electric device and methods for
operating the device) patent application- Launched aesthetic plasma device PLAMERE
- Launched PDRN cosmetic Clapio-Cell
- Launched mesotherapy cosmetic Clapio-Cell, Clapio-Vitamin,
Clapio-Glutathione- Permit for manufacturing of cosmetic products

2016
- GCS Co., Ltd. R&D
(Company Annex Research Institute, Venture Business Confirmation, Confirmation of ISO13485)
2015
- Completed medical device manufacturing permit and item permit, application for patent of GANA Fill
- Manufacture and sales of electric high-frequency device Freespot / KGMP approval
- Manufacture and sales of Art Aqua (Meso solution)
- Manufacture and sales of ART Injector

2014
- Overseas launch of GANA FILL (Graft and prosthesis)

2013
- Received medical-use Lifting Thread ‘Art Lift’ CE Certification
- Manufacture and distribution of beauty device RF Needling System
- Patent application registration (medical image processing equipment and methods for medical image standards)
2012
- Acquired medical device GMP
- Acquired cosmetic product manufacturing GMP
- Acquired ISO13485
- Launched Art Lift (tissue-fixing thread)

2011
- Establishment of GCS Co., Ltd
- Obtained permit to import medical devices
- Imports and distribution of medical bandage (CoFelx), a distributor contract for Korean / Japanese agents
- Established branch office in Japan
- Ministry of Knowledge Economy (Business Cooperation with Korea Technology Transfer Agents Association)
- Technical transaction registration No. 2011-0141
- Received ISO 9001/14001